February 2024
One of the central goals in the PULS group is the knowledge-based design of new selective and highly-active catalytic systems. To achieve this goal, we utilize state of the art (hybrid) quantum mechanical and molecular mechanical methods to predict the physico-chemical properties and underlying reaction mechanisms of the newly designed catalysts.
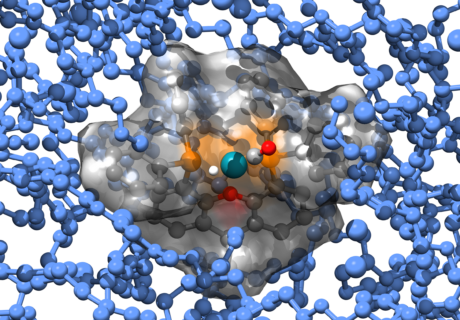
One of the most promising catalytic systems investigated in our group are so-called Supported Ionic Liquid Phase (SILP) catalysts. SILP catalysts consist of an ionic liquid film containing a catalytically active homogeneous transition metal complex (Figure), which is further immobilized on a (highly porous) solid support. Due to the extremely low vapor pressure of the IL, the final SILP material is still a macroscopically dry and free flowing material.
Using our in silico techniques, we can investigate the reaction mechanisms of the catalysts targeting different reaction types (e.g. water-gas-shift, hydrogenation, dehydrogenation, hydroformylation, etc.), the influence of inorganic and organic additives on the catalytic activity and predict the positioning of the catalytic species within the film via knowledge based ligand design.
The image shows a snapshot from one of our Molecular Dynamics simulations of a xantphos-based hydroformylation catalyst solvated in a functionalized bis-(mPEG)-imidazolium based ionic liquid at the molecular scale (size of the systems: 10-100 nm). These simulations provide detailed insights into the processes dictating the positioning of the catalysts within the ionic liquid film and into the molecular details of the reaction mechanisms incorporating the influence of the solvent.
Link to Research Group:
Physics Underlying Life Sciences
Example Publications:
https://doi.org/10.1021/acscatal.1c05979
Wolf, P.; Wick, C. R.; Mehler, J.; Blaumeiser, D.; Schötz, S.; Bauer, T.; Libuda, J.; Smith, D.; Smith, A.-S.; Haumann, M. Improving the Performance of Supported Ionic Liquid Phase Catalysts for the Ultra-Low-Temperature Water Gas Shift Reaction Using Organic Salt Additives. ACS Catal. 2022, 12 (9), 5661–5672.
https://doi.org/10.1002/anie.201811627
Stepić, R.; Wick, C. R.; Strobel, V.; Berger, D.; Vučemilović‐Alagić, N.; Haumann, M.; Wasserscheid, P.; Smith, A.-S.; Smith, D. M. Mechanism of the Water–Gas Shift Reaction Catalyzed by Efficient Ruthenium-Based Catalysts: A Computational and Experimental Study. Angew. Chem., Int. Ed. Engl. 2019, 58 (3), 741–745.
https://doi.org/10.1016/j.jil.2022.100041.
Seidl, V.; Bosch, M.; Paap, U.; Livraghi, M.; Zhai, Z.; Wick, C. R.; Koller, T. M.; Wasserscheid, P.; Maier, F.; Smith, A.-S.; Bachmann, J.; Steinrück, H.-P.; Meyer, K. Bis-Polyethylene Glycol-Functionalized Imidazolium Ionic Liquids: A Multi-Method Approach towards Bulk and Surface Properties. Journal of Ionic Liquids 2022, 2 (2), 100041.
